Twelve Hallmarks of Ageing
The paper titled “Twelve Hallmarks of aging” published in the journal Cell proposes a framework for understanding the complex biological processes that underlie ageing. Ageing is a natural phenomenon that affects all organisms, and is associated with a gradual decline in physiological function and increased vulnerability to age-related diseases. While the exact mechanisms of ageing remain poorly understood, recent advances in molecular and cellular biology have led to a growing appreciation of the complex network of pathways and processes that contribute to ageing.
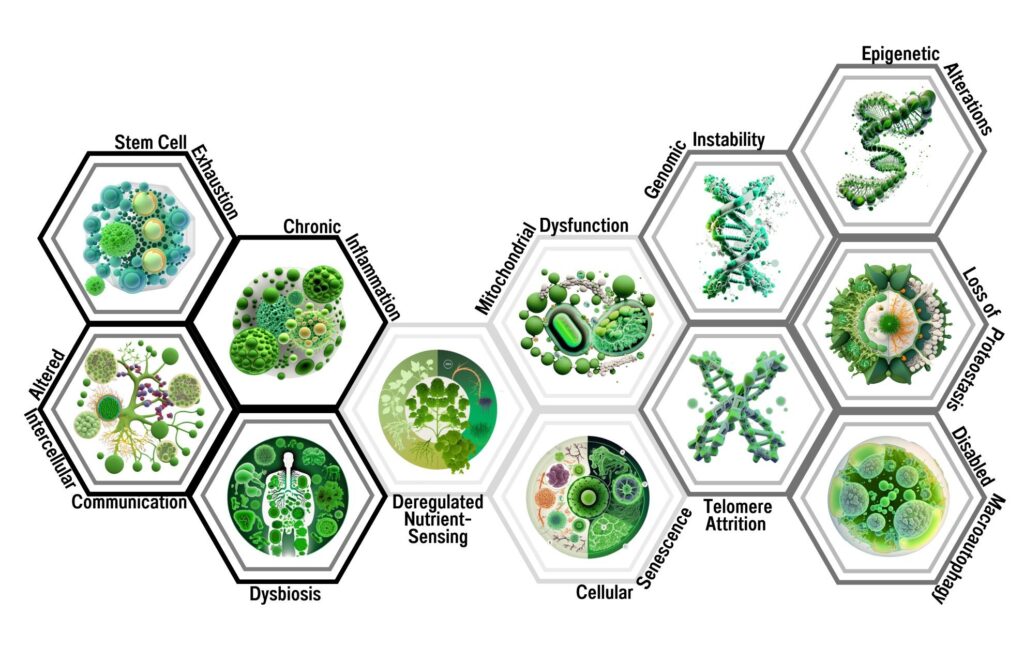
The authors of this paper propose a comprehensive framework for understanding the interconnected biological mechanisms underlying ageing. The paper identifies twelve hallmarks that collectively contribute to the ageing process: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis.
Each of these hallmarks represents a distinct biological mechanism that contributes to the ageing process, and they are all interconnected and influence each other. For example, genomic instability can lead to mutations and chromosomal rearrangements that affect gene expression and cellular function, which can in turn lead to loss of proteostasis and mitochondrial dysfunction. Similarly, telomere attrition can contribute to stem cell exhaustion and cellular senescence, which can lead to altered intercellular communication and chronic inflammation.
Understanding the mechanisms underlying these hallmarks of ageing is essential for developing interventions to slow or even reverse the ageing process and reduce the risk of age-related diseases. By addressing each of these hallmarks, researchers may be able to identify effective strategies to extend healthspan and improve overall health and well-being in older adults.
By identifying and describing these hallmarks of ageing, the authors hope to provide a more complete understanding of the biological processes that contribute to ageing, as well as potential targets for interventions to slow or even reverse the ageing process. This framework may also provide insights into the underlying mechanisms of age-related diseases, and inform the development of new treatments for these conditions. Overall, the paper represents a significant contribution to the field of ageing research, and provides a valuable roadmap for future investigations into the complex biology of ageing.
Brief Introduction on 12 Hallmarks
The ageing process is a complex and multifactorial phenomenon that involves a gradual decline in the functional capacity of various organs and systems. In recent years, several studies have suggested that ageing is driven by a variety of molecular and cellular processes that contribute to the progressive deterioration of cellular function and tissue homeostasis. To better understand the molecular basis of ageing, a comprehensive framework that integrates different cellular processes has been proposed. This framework, known as the hallmarks of ageing, describes a set of interconnected cellular and molecular processes that contribute to the ageing process.
The hallmarks of ageing are a set of 12 biological processes that are thought to contribute to the ageing process. These hallmarks include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis. Each of these hallmarks is interconnected and contributes to the ageing process by affecting various cellular pathways and functions.
Genomic instability is a hallmark of ageing that refers to the accumulation of DNA damage and mutations in cells. This can lead to chromosomal aberrations, such as deletions, translocations, and inversions, which can contribute to cellular dysfunction and disease.
Telomere attrition refers to the progressive shortening of telomeres, the protective caps at the ends of chromosomes. This can lead to cellular senescence, which is a state of irreversible growth arrest, and contribute to the ageing process.
Epigenetic alterations refer to changes in the regulation of gene expression that occur without alterations to the underlying DNA sequence. These changes can be driven by environmental factors, such as diet and lifestyle, and contribute to the ageing process.
Loss of proteostasis refers to the failure of cells to properly maintain the folding and degradation of proteins. This can lead to the accumulation of misfolded and damaged proteins, which can contribute to cellular dysfunction and disease.
Disabled macroautophagy refers to the impairment of a cellular process that involves the degradation of damaged organelles and proteins. This can lead to the accumulation of damaged organelles and proteins, which can contribute to cellular dysfunction and disease.
Deregulated nutrient-sensing refers to the disruption of cellular pathways that regulate energy metabolism and nutrient availability. This can lead to the accumulation of excess nutrients and energy, which can contribute to cellular dysfunction and disease.
Mitochondrial dysfunction refers to the impairment of cellular organelles that are responsible for energy production. This can lead to a reduction in cellular energy production and an increase in the production of reactive oxygen species, which can contribute to cellular dysfunction and disease.
Cellular senescence refers to a state of irreversible growth arrest that can be triggered by various stressors, such as DNA damage or telomere attrition. Cellular senescence can contribute to the ageing process by reducing tissue regenerative capacity.
Stem cell exhaustion refers to the depletion of the pool of stem cells, which are responsible for tissue regeneration and repair. This can lead to a reduction in tissue regenerative capacity and contribute to the ageing process.
Altered intercellular communication refers to changes in the signaling between cells, which can affect tissue homeostasis and function. These changes can contribute to the ageing process by impairing tissue regenerative capacity and promoting tissue dysfunction.
Chronic inflammation refers to a sustained and low-grade inflammatory response that can occur in various tissues and organs. This can contribute to the ageing process by promoting tissue dysfunction and disease.
Dysbiosis refers to changes in the composition and function of the microbiota, the diverse population of microorganisms that live in and on the human body. Dysbiosis can contribute to the ageing process by promoting inflammation and impairing immune function.
Understanding these 12 hallmarks of ageing is crucial for developing interventions that can prevent or reverse the ageing process and improve healthspan in ageing individuals.
First Hallmark: Genomic instability
The paper “Twelve Hallmarks of ageing” published in the journal Cell identifies and describes twelve interconnected hallmarks that collectively contribute to the ageing process. The first hallmark is genomic instability, which refers to the accumulation of mutations and DNA damage over time.
Genomic instability can arise from a variety of sources, including exposure to environmental toxins, radiation, and errors in DNA replication and repair. As cells divide and age, they are increasingly susceptible to DNA damage, which can result in mutations and chromosomal rearrangements that can affect gene expression and cellular function. This can contribute to the ageing process by disrupting cellular and tissue function, increasing the risk of cancer and other age-related diseases, and ultimately leading to cellular senescence and death.
The authors of the paper highlight the importance of genomic instability as a key driver of ageing and age-related diseases. They note that understanding the mechanisms underlying genomic instability is critical for developing interventions to slow or even reverse the ageing process. They also suggest that genomic instability may be a potential target for cancer therapies, as many cancers arise from mutations and chromosomal abnormalities that result from genomic instability.
The authors discuss a number of pathways and mechanisms that contribute to genomic instability, including errors in DNA replication, exposure to reactive oxygen species, and defects in DNA repair pathways. They also note that genomic instability can be influenced by a variety of genetic and environmental factors, including lifestyle choices such as diet and exercise.
Overall, the first hallmark of ageing, genomic instability, highlights the central role that DNA damage and mutations play in the ageing process. By understanding the underlying mechanisms of genomic instability, researchers may be able to develop interventions to slow or even reverse the ageing process and reduce the risk of age-related diseases.
Second Hallmark: Telomere attrition
The second hallmark of ageing identified in the paper “Twelve Hallmarks of ageing” published in the journal Cell is telomere attrition. Telomeres are specialized structures located at the ends of chromosomes that protect the genetic material from damage during cell division. Telomeres shorten with each cell division, and as they approach a critical length, cells enter a state of replicative senescence, or cellular ageing, and can no longer divide.
Telomere attrition is a key contributor to the ageing process and is associated with the development of age-related diseases such as cardiovascular disease, osteoporosis, and cancer. The exact mechanisms underlying telomere attrition and its effects on cellular function are still being studied, but it is thought that telomere shortening contributes to cellular dysfunction and senescence by altering gene expression, disrupting DNA repair mechanisms, and activating pro-inflammatory pathways.
The authors of the paper propose that interventions to prevent or slow telomere attrition may have therapeutic potential for treating age-related diseases and extending healthspan. Such interventions could include telomerase activation, which lengthens telomeres and has been shown to increase cellular lifespan in vitro, or lifestyle modifications such as exercise, stress reduction, and healthy diet, which have been associated with longer telomere length in humans.
Overall, the second hallmark of ageing, telomere attrition, highlights the importance of telomeres in maintaining genomic stability and cellular function, and their gradual erosion as a key driver of the ageing process. By understanding the underlying mechanisms of telomere attrition, researchers may be able to develop interventions to slow or even reverse the ageing process and reduce the risk of age-related diseases.
Third Hallmark: Epigenetic alterations
The third hallmark of ageing identified in the paper “Twelve Hallmarks of ageing” published in the journal Cell is epigenetic alterations. Epigenetics refers to changes in gene expression that are not caused by alterations to the DNA sequence itself, but rather modifications to the proteins and chemical tags that interact with the DNA. These modifications can affect the structure of chromatin and the accessibility of DNA to transcription factors, which can either promote or repress gene expression.
Epigenetic alterations have been linked to a range of age-related diseases, including cancer, cardiovascular disease, and neurodegeneration. As we age, there is a gradual loss of epigenetic stability, with changes in DNA methylation patterns, histone modifications, and alterations in the expression of non-coding RNAs. These changes can lead to a loss of cellular identity, impaired tissue homeostasis, and increased cellular senescence.
The authors of the paper note that epigenetic alterations are highly dynamic and can be influenced by a variety of factors, including environmental exposures, diet, and lifestyle choices. They also suggest that interventions aimed at restoring epigenetic stability may have potential for slowing or even reversing the ageing process and reducing the risk of age-related diseases.
Overall, the third hallmark of ageing, epigenetic alterations, highlights the critical role that epigenetics plays in regulating gene expression and maintaining cellular identity. By understanding the underlying mechanisms of epigenetic alterations and identifying potential interventions to restore epigenetic stability, researchers may be able to develop new approaches to promoting healthy ageing and reducing the risk of age-related diseases.
Fourth Hallmark: The loss of proteostasis
The fourth hallmark of ageing identified in the paper “Twelve Hallmarks of ageing” is the loss of proteostasis, which refers to the gradual decline in the body’s ability to maintain proper protein folding and degradation. Proteins are essential molecules that perform a wide range of functions within cells, and their proper folding and degradation are critical for maintaining cellular health and function.
Proteostasis involves a complex network of cellular pathways that regulate protein synthesis, folding, and degradation. However, with ageing, this network becomes less efficient, leading to an accumulation of misfolded and damaged proteins. These proteins can form aggregates that disrupt cellular function, leading to a variety of age-related diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s.
The loss of proteostasis can arise from a variety of factors, including oxidative stress, inflammation, and impaired autophagy, which is the process by which cells recycle damaged proteins and organelles. As cells age, they become less able to manage the accumulation of damaged proteins, which can lead to the formation of toxic aggregates that can impair cellular function.
Understanding the underlying mechanisms of proteostasis and the factors that contribute to its decline is crucial for developing interventions to slow or even reverse the ageing process. Researchers are exploring various strategies to enhance proteostasis, including the use of small molecules that promote protein folding and clearance, as well as dietary interventions that support healthy ageing. Ultimately, developing effective interventions to maintain proteostasis may help prevent or delay the onset of age-related diseases and improve overall healthspan.
Fifth Hallmark: Disabled macroautophagy
Disabled macroautophagy is one of the twelve hallmarks of ageing, as proposed in the paper “The Hallmarks of ageing 2.0” published in Cell in 2022. Macroautophagy is a highly conserved cellular process that plays a critical role in the maintenance of cellular homeostasis by degrading damaged organelles, misfolded proteins, and other cellular debris. Disabled macroautophagy occurs when this process is impaired or disrupted, leading to the accumulation of damaged organelles and proteins in cells, which can contribute to cellular dysfunction and disease.
In recent years, there has been growing interest in the role of macroautophagy in ageing and age-related diseases. Studies have shown that macroautophagy declines with age in various organisms, including humans. This decline has been linked to the accumulation of damaged organelles and proteins, which can contribute to cellular dysfunction and disease.
Disabled macroautophagy has been linked to a variety of age-related diseases, including neurodegenerative diseases, cancer, and metabolic disorders. In neurodegenerative diseases, impaired macroautophagy has been linked to the accumulation of toxic protein aggregates, such as amyloid-beta and tau, which are associated with Alzheimer’s and Parkinson’s diseases. In cancer, impaired macroautophagy has been linked to the accumulation of damaged mitochondria, which can contribute to the development and progression of cancer. In metabolic disorders, impaired macroautophagy has been linked to the accumulation of lipid droplets and the development of insulin resistance.
Recent research has also suggested that macroautophagy may play a role in the regulation of the ageing process itself. Studies have shown that interventions that enhance macroautophagy, such as caloric restriction and exercise, can extend lifespan in various organisms. These findings have led to growing interest in the development of interventions that target macroautophagy to prevent or reverse the ageing process and age-related diseases.
In conclusion, disabled macroautophagy is a hallmark of ageing that contributes to cellular dysfunction and disease by impairing the cellular process of macroautophagy. Understanding the role of macroautophagy in ageing and age-related diseases may lead to the development of new interventions to prevent or reverse these conditions.
Sixth Hallmark: Deregulated nutrient sensing
The sixth hallmark of ageing, as proposed in the paper “Twelve Hallmarks of ageing,” is deregulated nutrient sensing. This hallmark refers to the changes that occur in the body’s ability to sense and respond to nutrient levels, particularly with regard to the regulation of growth and metabolism.
In normal physiology, the body’s nutrient-sensing mechanisms play a critical role in maintaining energy balance, regulating growth and development, and responding to changes in nutrient availability. However, as we age, these mechanisms can become less efficient, leading to dysregulation of nutrient sensing.
One of the key drivers of deregulated nutrient sensing is insulin resistance, a condition in which the body becomes less responsive to the hormone insulin, which is involved in the regulation of glucose metabolism. Insulin resistance is a hallmark of ageing and is associated with increased risk for type 2 diabetes, cardiovascular disease, and other age-related disorders.
Other factors that can contribute to deregulated nutrient sensing include alterations in the activity of growth hormone and the insulin-like growth factor 1 (IGF-1) signaling pathway, which play key roles in regulating growth and metabolism. Dysregulation of these pathways can lead to changes in nutrient partitioning, altered metabolism, and increased risk for age-related diseases.
Understanding the mechanisms underlying deregulated nutrient sensing is essential for developing interventions to slow or reverse the ageing process and reduce the risk of age-related diseases. Potential interventions include dietary and lifestyle interventions, as well as the development of new drugs that target specific nutrient-sensing pathways.
Seventh Hallmark: Mitochondrial dysfunction
The seventh hallmark of ageing identified by the authors of the paper “Twelve Hallmarks of ageing” is mitochondrial dysfunction. Mitochondria are organelles responsible for generating energy in the form of ATP through oxidative phosphorylation. They also play important roles in cellular signaling, apoptosis, and calcium homeostasis. As cells age, mitochondrial function can deteriorate, leading to reduced energy production and increased oxidative stress. This can cause cellular damage, DNA mutations, and contribute to the ageing process.
Mitochondrial dysfunction can arise from a variety of factors, including genetic mutations, environmental stressors, and changes in cellular metabolism. For example, accumulation of mtDNA mutations can result in impaired respiratory chain function and increased reactive oxygen species production. Additionally, changes in nutrient availability and metabolism, such as those seen in caloric restriction or high-fat diets, can also affect mitochondrial function and contribute to ageing.
Mitochondrial dysfunction has been linked to a range of age-related diseases, including neurodegenerative disorders, cardiovascular disease, and cancer. Understanding the mechanisms underlying mitochondrial dysfunction is crucial for developing interventions to slow or even reverse the ageing process and reduce the risk of age-related diseases. Potential strategies include targeting mitochondrial biogenesis and turnover, reducing oxidative stress, and improving mitochondrial quality control mechanisms.
Eighth Hallmark: Cellular senescence
Cellular senescence is the process by which cells cease to divide and enter a state of permanent cell cycle arrest. While senescence is a normal cellular response to stress or damage, it also plays a critical role in the ageing process. In the recent paper titled “Twelve Hallmarks of ageing,” cellular senescence is identified as one of the key hallmarks of ageing.
As cells undergo senescence, they exhibit a range of phenotypic changes, including alterations in gene expression, chromatin structure, and metabolism. These changes can have significant consequences for tissue function, particularly in tissues with a high cell turnover rate such as skin, gut, and blood. Additionally, senescent cells secrete a range of pro-inflammatory cytokines, chemokines, and growth factors, collectively known as the senescence-associated secretory phenotype (SASP). The SASP can promote inflammation and tissue dysfunction, and is thought to contribute to age-related diseases such as cancer, diabetes, and cardiovascular disease.
Cellular senescence is triggered by a range of stressors, including DNA damage, telomere shortening, and activation of oncogenes. The senescence response is mediated by the p53 and p16INK4a pathways, which induce cell cycle arrest and promote senescence-associated phenotypic changes. While senescence is initially a protective response that prevents damaged cells from proliferating and contributing to tumor formation, the accumulation of senescent cells over time can have deleterious effects on tissue function and contribute to the ageing process.
The identification of cellular senescence as a hallmark of ageing has significant implications for the development of interventions to slow or even reverse the ageing process. Strategies to selectively eliminate senescent cells, known as senolytics, have been shown to improve tissue function and extend healthspan in animal models. Additionally, targeting the SASP may offer a promising approach to treating age-related diseases associated with inflammation and tissue dysfunction.
Ninth Hallmark: Stem cell exhaustion
The ninth hallmark of ageing, as proposed by the paper “Twelve Hallmarks of ageing,” is stem cell exhaustion. Stem cells are specialized cells that have the ability to self-renew and differentiate into various cell types in the body. They play a crucial role in tissue repair, regeneration, and maintenance throughout an organism’s lifespan. However, as an organism ages, the regenerative capacity of stem cells declines, leading to impaired tissue function and increased susceptibility to age-related diseases.
Stem cell exhaustion can occur through various mechanisms. One possible mechanism is the depletion of stem cell pools due to continuous differentiation or activation in response to tissue damage or chronic inflammation. Another mechanism is the accumulation of DNA damage and epigenetic changes in stem cells, which can impair their self-renewal and differentiation potential.
As stem cell exhaustion progresses, the body’s ability to regenerate and repair tissues diminishes, leading to various age-related pathologies, including muscle wasting, neurodegeneration, and cardiovascular disease. Understanding the mechanisms underlying stem cell exhaustion and developing interventions to enhance stem cell function and regeneration are critical for extending healthy lifespan and reducing the burden of age-related diseases.
Tenth Hallmark: Altered intercellular communication
Altered intercellular communication is the eleventh hallmark of ageing, as proposed in the paper “The Hallmarks of ageing 2.0” published in Cell in 2022. Intercellular communication refers to the exchange of information and signals between cells, which is essential for the proper functioning of tissues and organs in the body. This communication occurs through various mechanisms, including direct cell-to-cell contact, secretion of signaling molecules such as hormones and growth factors, and the release of extracellular vesicles.
As the body ages, intercellular communication becomes dysregulated, which can contribute to various age-related diseases and conditions. Altered intercellular communication can occur through various mechanisms, such as changes in the production or reception of signaling molecules, changes in cell surface receptors, and changes in the extracellular matrix.
Recent research has highlighted the role of altered intercellular communication in the development of age-related diseases, such as cancer, neurodegeneration, and chronic inflammation. In cancer, altered intercellular communication can contribute to the development and progression of the disease by promoting tumor cell growth and survival. In neurodegeneration, altered intercellular communication can contribute to the accumulation of toxic proteins and the death of neurons. In chronic inflammation, altered intercellular communication can contribute to the sustained activation of immune cells and tissue damage.
Moreover, altered intercellular communication can also lead to changes in stem cell behavior, contributing to the decline in tissue regenerative capacity with age. For example, in muscle tissue, ageing-related changes in intercellular communication have been shown to impair the regenerative capacity of satellite cells, leading to reduced muscle repair and regeneration.
The development of new therapeutic interventions that target altered intercellular communication is an emerging area of research in ageing and age-related diseases. These interventions could potentially restore proper communication between cells and tissues, improving tissue function and preventing or reversing age-related diseases.
In conclusion, altered intercellular communication is a hallmark of ageing that contributes to the dysregulation of cellular and tissue functions, leading to various age-related diseases and conditions. Understanding the mechanisms underlying altered intercellular communication in ageing and age-related diseases may lead to the development of new interventions to improve tissue function and prevent or reverse age-related diseases.
Eleventh Hallmark: Chronic inflammation
Chronic inflammation is a hallmark of ageing and a key contributor to age-related diseases such as cancer, cardiovascular disease, and neurodegeneration. Inflammation is a natural response of the immune system to injury or infection, but chronic inflammation occurs when this response becomes dysregulated and persistent. The eleventh hallmark of ageing, chronic inflammation, is characterized by elevated levels of pro-inflammatory cytokines and immune cells that contribute to tissue damage and dysfunction.
As we age, our immune system undergoes changes, including a decline in the function of immune cells, called T cells and B cells, and an increase in the production of pro-inflammatory cytokines. This shift towards chronic inflammation can be triggered by a variety of factors, including chronic infections, exposure to environmental toxins, and a diet high in processed and sugary foods. Chronic inflammation can also be exacerbated by cellular stress, such as DNA damage or cellular senescence.
Chronic inflammation contributes to the ageing process by promoting the accumulation of damaged cells and tissues, impairing tissue repair and regeneration, and contributing to the development of age-related diseases. In addition, chronic inflammation has been linked to cognitive decline, depression, and other age-related declines in physical and mental health.
Understanding the mechanisms underlying chronic inflammation is essential for developing interventions to slow or even reverse the ageing process and reduce the risk of age-related diseases. By targeting specific pro-inflammatory cytokines or immune cells, researchers may be able to develop therapies that can modulate chronic inflammation and improve healthspan in ageing individuals.
Twelfth Hallmark: Dysbiosis
The twelfth hallmark of ageing, dysbiosis, refers to the disruption of the microbial communities that inhabit the gut, skin, and other tissues in the body. Dysbiosis is an imbalance in the composition and function of the microbiome, which can lead to a range of negative health outcomes, including chronic inflammation, metabolic dysfunction, and an increased risk of infectious diseases.
The microbiome is a complex ecosystem of microorganisms, including bacteria, viruses, fungi, and other organisms, that play a critical role in maintaining the health and function of the human body. These microorganisms perform a variety of functions, including aiding in digestion, producing vitamins, and regulating the immune system. The composition and diversity of the microbiome are influenced by factors such as diet, environmental exposure, and host genetics.
Dysbiosis can occur as a result of various factors, including the use of antibiotics, dietary changes, and exposure to environmental toxins. In turn, dysbiosis can lead to the depletion of beneficial microorganisms and the overgrowth of harmful ones, disrupting the delicate balance of the microbiome. This disruption can result in chronic inflammation, which is associated with a range of age-related diseases, including diabetes, cancer, and cardiovascular disease.
Understanding the role of dysbiosis in the ageing process is crucial for developing interventions to promote healthy ageing. Interventions such as prebiotics, probiotics, and dietary modifications can help to restore the balance of the microbiome and promote healthy ageing. Additionally, targeting dysbiosis may have implications for the treatment and prevention of age-related diseases, making it an important area of research in the field of ageing.